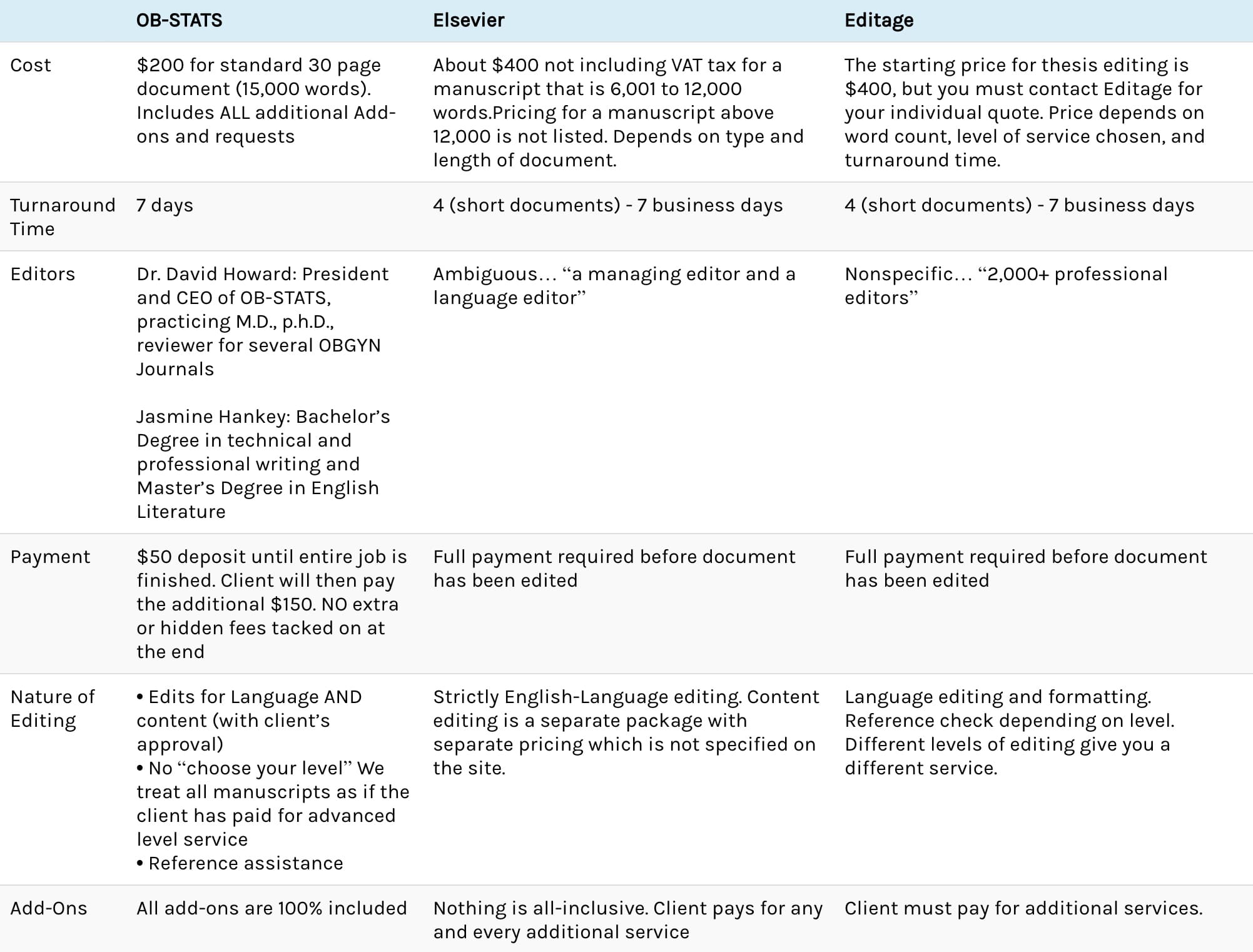
Types of Research Study Designs
Types of Research Study Designs
After I have given a great deal of thought to what I will be researching, I begin organizing my study based upon the specific study objective I came up with during my brainstorming process. When designing a study, it is important to choose the correct research study design based upon the research objective you are investigating. This blog looks at the different study designs, their limitations, and benefits.
Experimental Study Designs
There are two types of experimental study designs: experimental studies and clinical trials.
- Experimental Studies—This type of study is used to test a hypothesis, not to cater to the subjects’ needs. However, it is important to remember that ethical limitations are crucial in this type of study. If you were studying sleep deprivation, where enforced deprivation could lead to subjects’ involuntary self-harm due to narcolepsy, you would want to be careful to protect the safety of your subjects.
- Clinical Trials—This type of study is used to evaluate potential cures or disease prevention in patients. Clinical trial treatment assignments should be assigned to minimize differences in influencing factors that might affect comparisons of each subject to the overall population. Making these assignments random can help to prevent causal inference. The validity of the study results depends on how comparable the treatment is to factors other than treatment itself. Blinding should be employed for treatment assignment and assessment.
Non-Experimental Study Designs
There are three types of non-experimental studies: cohort, case control, and cross-sectional studies.
- Cohort Studies—In a cohort study, at least two groups of people are determined, and different cohorts have different exposures to potential cause of disease. One group is the “exposed cohort” and the other is the “reference cohort.”
- Case Control Studies—A case control study is a more efficient version of cohort study. Here, subjects (or “cases”) are identified by whether they have the disease or condition. Controls are subjects who do not have the disease, but subjects are sampled independently of exposure status.
- Cross-Sectional Study—This study may be purely descriptive in nature. The exposure and outcome are determined simultaneously, and the contrast is to the case control. Limitations of cross-sectional studies include over representation of cases with long durations of illness and under representation of those with short durations.

Choosing A Research Study Design
When choosing a study design, it is important to remember that some research questions can be answered by multiple different study designs, so it is crucial to match your study objective with the design that will best return the kind of results you want.
For example, say our study objective was to determine whether taking vitamins during early pregnancy can reduce nervous system defects in a developing fetus. In this case, I would choose a study design that determines incidence, or the number of new cases of a disease over a specific timespan. The best choice of study design would be either a randomized clinical trial or a cohort study, since both designs start with people who do not have the disease and follow them over time until they develop a disease.
For example, if I wanted to know whether a treatment can reduce the incidence of a disease, or to know with certainty that something causes a certain outcome, I would choose a controlled experiment, such as a randomized controlled trial. Or, if I wanted to determine causation, I could choose an experimental study design.
If my study objective was to determine whether taking vitamins during early pregnancy is associated with (rather than can reduce) nervous system defects in a developing fetus, I would choose a study design that can determine association, such as a non-experimental study design.
Overall, for this study objective, the researcher could use an observational study design, such as a cohort study, but, as can be seen from the previous examples, the study design is dependent upon the study objectives. As you can see, knowing your study objective is extremely important for choosing which study design you will use.
Learn About “How To Do A Research Project” By OB-STATS
“How To Do A Research Project” was developed by Dr. David Howard. Having earned both a phD and MD, he is knowledgeable and passionate about research. He is devoted to creating Continuing Medical Education (CME) content that is useful to researchers and practitioners in the medical field. Dr. Howard’s most recent course will walk you through every step of the research project process and earn you CME credit. If you’re interested in the course, click below or visit Obstats.com.